Hot STM Labs
![]()
A Metastable Initial State in Benzene Adsorption
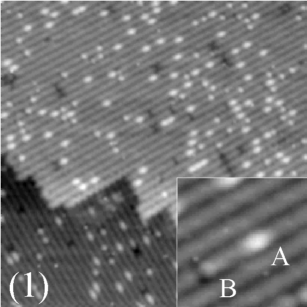
(400 Å x 400 Å)
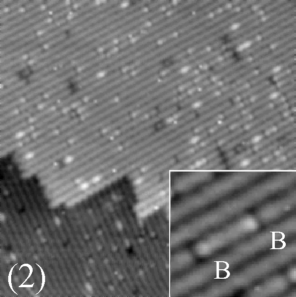
(400 Å x 400 Å)
Comparison with Theory
Experimental Observations |
|
(40 Å x 40 Å) |
|
State A
|
State
B
|
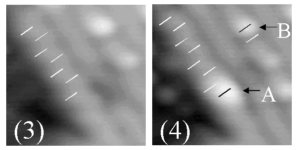
| In Figures 3 and 4, the locations of individual, buckled, substrate dimers are marked by white dashes. The black dash marking the center of the state A benzene atom in Fig 4 maps onto a substrate dimer. The separation between the light and dark halves of the state B benzene molecule in Fig 4 is equal to the separation of substrate dimers, (3.84 Å.) | |
Theoretical Models
| Correspondence to STM images | |||
Pedestal
|
(not observed) | 0 eV |
|
Butterfly:
|
State A |
- 1.39 eV |
|
Tilt
|
State B |
Proposed by |
|
The high energy Pedestal state proposed by Birkenheuer, Gutdeutsch, and Rosch1 is not observed, however the Butterfly state's symmetry and placement over a dimer suggest its identification as the observed state A. A good candidate for the observed state B is the Tilt state proposed by Lopinski, Fortieer, Moffatt, and Wolkow2. The asymmetry and placement of the benzene in the Tilt state agrees with that of state B. Another appeal of the Tilt state is that a conversion from the Butterfly state is easily conceived. On a heated surface, the benzene molecule adsorbed in the Butterfly state could wobble, and bond to an adjacent dimer to form the Tilt state. While semi-empirical calculations have been performed for these and other proposed states, only the functional density calculations, as performed by Birkenheuer, Gutdeutsch, and Rosch and Lopinski1 and Fortieer, Moffatt, and Wolkow3 find the unobserved pedestal configuration unstable.
1Gutdeutsch, and Rosch and Lopinski. Surface Science.
2Lopinski, Fortieer, Moffatt, and Wolkow. JVST A.
3Wolkow, Lopinski, and Moffatt. Work in progress.
Copyright 1998 by the Regents of the University of Minnesota, Dept. of Physics & Astronomy. All rights reserved.